Abstract
Acute lymphoblastic leukemia (ALL) is the most common malignancy in children and adolescents. Children with Down syndrome (trisomy 21) have a 30-times higher risk of acquiring ALL and pose up to 5% of all pediatric ALL patients. Moreover, many Down syndrome-ALL patients (DS-ALL) suffer from severe toxicity during chemotherapy, especially after application of high dose methotrexate (HD-MTX). Severe toxicities often result in MTX dose reduction, which may be associated with a higher probability of relapse. Systematic and comprehensive toxicity data in a large cohort of uniformly treated DS-ALL patients are lacking.
In order to extend our knowledge on MTX-associated toxicities in DS-ALL, we analyzed clinical data from 103 DS-ALL and 1109 Non-DS-ALL (NDS-ALL) patients diagnosed between 1995 and 2016 treated according to German ALL-BFM protocols (ALL-BFM 1995, ALL-BFM 2000 and AIEOP-BFM ALL 2009). We only included patients for whom both therapy and toxicity data were available. We focused on toxicity after HD-MTX administration during the 8-week HD-MTX consolidation in which patients receive 4 courses of intravenous HD-MTX (5 g/m2 each) plus intrathecal MTX in addition to 6-mercaptopurine (25 mg/m2/d). As of 2004, it was recommended for DS-ALL to administer the first MTX course with a reduced dose of 0.5 g/m2 and subsequently increase the dose if no severe toxicity occurs. Toxicity grading was performed according to CTC 2.0.
From the 103 DS-ALL patients four switched to high risk treatment and in one patient only incomplete data on toxicity were available. For these patients data could not be analyzed throughout the complete consolidation therapy. From the remaining 98 patients, 42 (43%) received MTX in a dose of 5 g/m2 ± 10%, six (6%) in a dose between 0.551 - 4.499 g/m2 and50 (51%) received a dose of 0.5 g/m2 ± 10% in the first MTX-block. In contrast, 1061 of 1109 (96%) NDS-ALL received an MTX dose of 5 g/m2.
One DS-ALL patient died due to a severe infection after the second MTX block and in two DS-ALL patients HD-MTX consolidation was stopped due to severe infections after the first and the third MTX block, respectively. In contrast, HD-MTX was stopped for two NDS-ALL patients due to toxicity (neurotoxicity and infection) after the second and third course, respectively. All five patients received a first MTX dose of 5 g/m2. No NDS-ALL patient died during HD-MTX therapy.
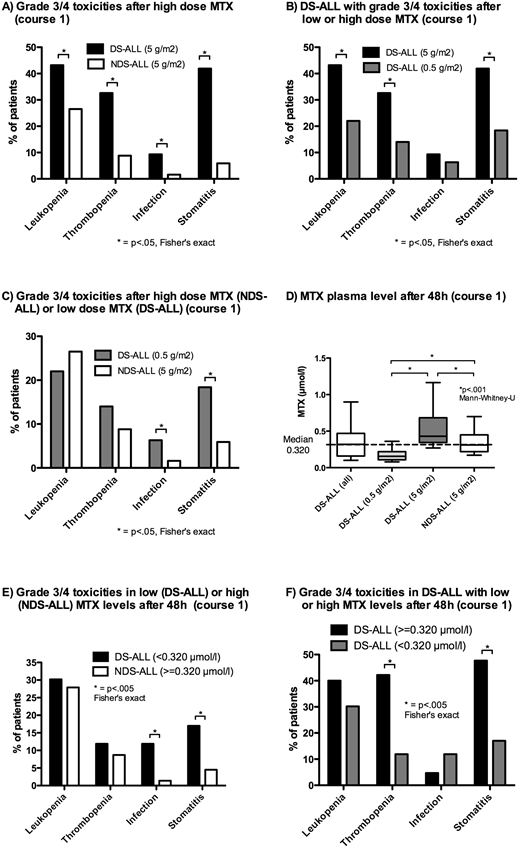
After receiving an MTX dose of 5 g/m2 DS-ALL showed significantly higher rates of grade 3/4 leukopenia, stomatitis, thrombocytopenia and infections as compared to NDS-ALL after the first course (Figure A). Reduction of the initial MTX dose to 0.5 g/m2 significantly reduced the rate of stomatitis, thrombocytopenia and leukopenia in DS-ALL by more than 40% (Figure B). However, these patients still suffered significantly more often from grade 3/4 stomatitis and infections compared to NDS-ALL who received full dose MTX (Figure C). Moderate MTX dose escalation for DS-ALL who tolerated a low MTX dose in the first course and received a higher MTX dose in the second course (median MTX dose 1 g/m2) did not result in an increased rate of toxicity as compared to the first course. Importantly, for DS-ALL a reduced MTX dose of 0.5 g/m2 in the first MTX block was not associated with a higher five year-cumulative risk of relapse (CIR) compared to a dose of 5 g/m2 (CIR 0.08±.05 vs. 0.16±0.05, p=.37).
Differences in MTX plasma levels at 42 h and 48 h after start of the MTX infusion did not explain the higher rate of toxicity in DS-ALL as compared to NDS-ALL as most DS-ALL patients who received a low MTX dose presented with significantly lower MTX plasma level than NDS-ALL controls who received high dose MTX (Figure D). Additionally, DS-ALL with MTX plasma levels lower than median level (<0.320 µmol/l) showed significantly higher rates of grade 3/4 stomatitis and infections than NDS-ALL with higher plasma levels (≥0.320 µmol/l, Figure E). Within the DS-ALL group high MTX plasma level were associated with significantly higher rates of grade 3/4 stomatitis and thrombocytopenia (Figure F).
In summary, MTX dose reduction in the first MTX course led to significantly decreased toxicity in DS-ALL without increasing the risk of relapse, although toxicity was still higher as compared to NDS-ALL. As low MTX plasma levels in DS-ALL were associated with less toxicity, lower cut-offs for higher leucovorine doses and forced diuresis may further reduce MTX toxicity in this highly vulnerable group of patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal